Xenotransplantation, a term denoting the transfer of non-human cells, tissues, or organs into humans for medical purposes, has emerged as a potential solution to the persistent shortage of organs available for transplantation. In the United States alone, an alarming average of seventeen individuals die each day while awaiting suitable organ donors. This pressing need underscores the importance of exploring alternative sources of organs to save lives.
Despite its promise, xenotransplantation remains an experimental approach, restricted to rare and critical cases pending further regulatory approval. The U.S. Food and Drug Administration (FDA) has yet to greenlight clinical trials, highlighting the necessity of comprehensive safety and efficacy assessments before widespread adoption.
The scope of xenotransplantation extends beyond vital organs like the kidney, heart, or liver, encompassing research into using animal cells or tissues to combat a range of serious conditions including epilepsy, chronic pain, Huntington’s disease, Parkinson’s disease, type 1 diabetes, and severe burns. However, for the purposes of this discussion, we will focus primarily on organ transplantation.
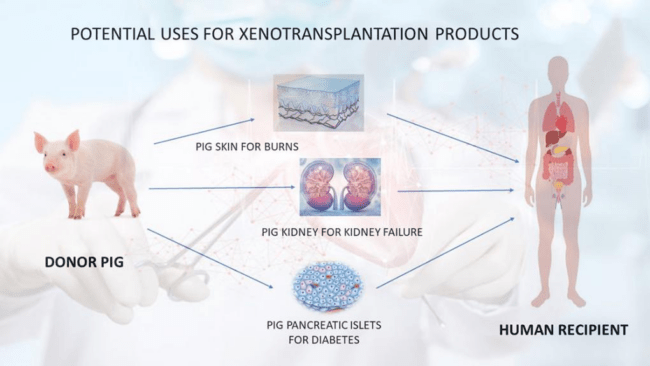
The mechanism behind xenotransplantation is multifaceted, with different methodologies employed depending on the target organ. In kidney transplants, for instance, the core principle involves transplanting a functional kidney from an animal into a human recipient. Pigs have emerged as the most promising candidates for providing organs due to their abundance, comparable kidney size to humans, and relatively low risk of disease transmission. Specially bred and genetically modified pigs are raised in controlled laboratory environments to enhance compatibility with human genes, thereby reducing the likelihood of rejection by the recipient’s immune system. Despite these advancements, recipients are still required to undergo post-transplantation immunosuppressive therapy to prevent rejection.
Decades of animal studies, predominantly involving the transplantation of pig organs into baboons due to their genetic similarity to humans, have provided valuable insights into the feasibility of cross-species organ transplantation. However, the transition to human clinical trials remains pending, with only a handful of operations conducted under exceptional circumstances with FDA approval and ethical oversight.
Safety concerns loom large in the realm of xenotransplantation, chief among them being the risk of infection inherent in all transplants. Animal organs may harbor pathogens specific to their species, potentially posing a greater infection risk than human organs. Additionally, the risk of organ rejection is heightened in xenotransplants due to genetic disparities between species. Efforts to mitigate this risk include genetic modifications in animals aimed at aligning their DNA more closely with that of humans. Nonetheless, questions persist regarding the transplanted organ’s ability to fully replicate the functions of its human counterpart.
A recent breakthrough in the field came in the form of the first successful transplant of a genetically modified pig kidney into a 62-year-old man suffering from end-stage kidney disease. Conducted by Surgeons and Nephrologists at the Mass General Transplant Center, the procedure utilized CRISPR-Cas9 technology to eliminate harmful pig genes and incorporate human genes to enhance compatibility. The patient, Richard “Rick” Slayman, is currently recuperating at Massachusetts General Hospital and is expected to be discharged soon. This landmark achievement not only offers hope to millions worldwide grappling with kidney failure but also represents a significant step forward in the quest for more readily available organs for transplantation.